Our Science backed Metabolic Protocol for Cancer Treatments address the multiple biological mechanisms cancer depends on. When used alongside conventional therapies like chemotherapy and radiation, it empowers your body to respond more intelligently, more effectively, and with renewed resilience — supporting natural healing and strengthening your defenses.
What We’ve Learned About Cancer Over the Years:
- Cancer cells aren’t just fast-growing — they’re metabolically rewired to consume energy differently from healthy cells.
- These altered energy pathways make cancer more resilient, more adaptive, and often more resistant to treatment over time.
- Modern research now confirms that metabolic targeting can play a critical role in supporting conventional treatments like chemotherapy, radiation, and immunotherapy.
Most cancer treatments focus on destroying cancer cells.
We complement that by asking: What does the cancer cell need to survive? And how can we take that away — systematically, and safely?
This is where Our Metabolic Protocols come in.
The Role of Metabolism in Cancer Progression & Recurrence
Cancer cells have unusually high energy needs to survive, grow, and spread. Normally, our body converts food into energy through a well-regulated process called metabolism, which supports cell function and repair. However, in cancer, this system often goes haywire. Cancer cells begin to use energy differently than healthy ones — a phenomenon that scientists now recognize as a key feature of the disease. This has given rise to a newer area of study called metabolic oncology, which focuses on how cancer cells fuel themselves. By understanding these unique metabolic changes, we can develop smarter, more targeted therapies.
Aerobic glycolysis, also known as the Warburg effect, is a clever survival mechanism that cancer cells rely on. Unlike normal cells, cancer cells continue producing energy rapidly even in the presence of oxygen — using this energy-hungry shortcut to fuel their aggressive growth, spread, and resistance to treatment.
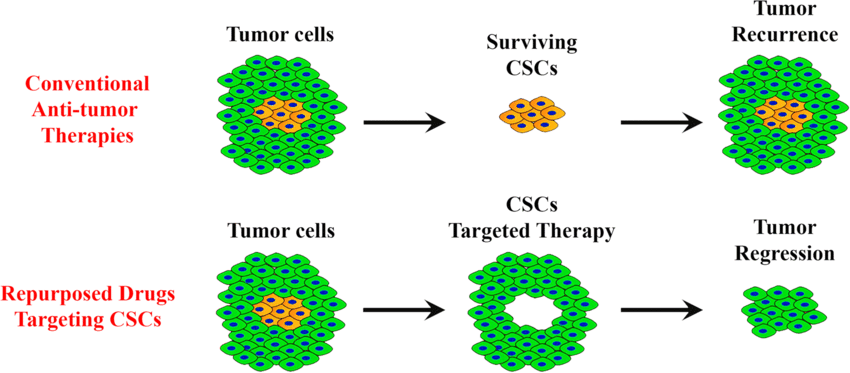
One of the biggest challenges in cancer treatment isn’t just shrinking the tumor — it’s preventing it from coming back. Cancer stem cells (CSCs) are a small but powerful group of cells that can survive conventional therapies like chemotherapy and radiation. They’re more adaptable, more resistant, and capable of regenerating the tumor even after most of it has been destroyed.
This is where the metabolic approach adds critical value.
CSCs depend on distinct metabolic pathways — different from the rest of the tumor mass. By understanding and targeting these unique energy systems, we can use a combination of repurposed drugs, natural compounds, and metabolic therapies to weaken CSCs without harming healthy cells.
When we disrupt the metabolic engine that fuels CSCs, we don’t just treat cancer — we reduce the chance it returns.This integrative strategy may lower recurrence risk and support longer-lasting remission — a direction increasingly supported by emerging research in metabolic oncology.
Our Metabolic Treatments are specifically designed to disrupt these altered metabolic pathways. By targeting the unique energy demands and vulnerabilities of cancer cells, the protocol helps restrict their energy supply and blocks alternate escape routes they might use to survive. This sustained metabolic stress can weaken cancer cells, making them more vulnerable to conventional treatments like chemotherapy, radiation, and other targeted therapies.
(Prolonged metabolic stress can significantly disrupt the internal energy machinery of cancer cells. As their metabolic rate drops, these cells begin to struggle with absorbing and utilizing essential nutrients from their surroundings — such as glucose, fats, and critical amino acids like glutamine and arginine. This nutrient deprivation weakens their ability to grow, multiply, and spread. Over time, it also reduces their adaptability to changes within the body, making it harder for them to survive and resist ongoing treatments).
Want to understand how this applies to your diagnosis?
Explore Our Science-backed Metabolic Protocol for your specific cancer type.
Selected Research Supporting Our Metabolic Protocol
Explore a curated selection of peer-reviewed studies that support our integrative cancer care philosophy.
- Doxycycline’s Impact on Tumor Burden in Breast Cancer Bone Metastasis (Duivenvoorden WC. (2002)) PMID: 11912125
- Link Between Metabolic Syndrome and Multiple Myeloma Risk Ragbourne S.C. et al. (2021) examined how metabolic dysregulation may influence the development of multiple myeloma. PMID: 32408305
- Exploring the Role of Metabolic Drugs in Hormone-Driven Cancers León-González A.J. et al. (2021) reviewed the potential of metformin and similar agents in the prevention and treatment of endocrine-related tumors, including prostate cancer. PMID: 34311387
- Targeting Cancer Stemness with Doxycycline: A 2021 study found that doxycycline may reduce the aggressive behavior of pancreatic cancer stem cells by disrupting key survival pathways (Liu H. et al., PMID: 33643917).
Cancer Pathways/ Mechanisms Targeted by Repurposed Drugs
| Cancer Pathway / Mechanism | Repurposed Drugs (PubMed / Research Reference) |
| Restrict Nutrient Supply to Tumor Cells | Célia Rosilio et al. 2014 PMID: 24462823, Melissa A Babcook et al. 2016. PMID: 27441003 |
| Induce Metabolic Stress in Cancer Cells | Ida Pernicova et al. 2014. PMID: 24393785, J.W. Clendening et al. 2012. PMID: 22310279, Borja Guerra et al. 2021 PMID: 33718197, Binlu Huang et al. 2020. PMID: 32694690, Grace H McGregor et al. 2020 PMID: 31562248, Gábor Petővári et al. 2018 PMID: 30574020 |
| Enhance the Immune System’s Response to Cancer Cells | Esraa AI Dujaily et al. 2020. PMID: 32190817, Yongjun Yi. et al. 2013. PMID: 23707077, Blom Kristin. et al. 2017 PMID: 28472897, Bahrambeigi Saman et al. 2019 PMID: 31884044, Eikawa Shingo et al. 2015. PMID: 25624476, Kurelac Ivana et al. 2019 PMID: 31091466, Lucero-Diaz P.A. et al. 2016 (Abstract), H Tang et al. 2013 PMID: 23282955 |
| Inhibit Cancer Cell Growth and Spread | Muhamad N.A.K. 2019. PMID: 31831021, Wen Jiang et al. 2021. PMID: 34303383, Tapas Mukhopadhyay 2002 PMID: 12231542, Laine C. Pinto et al. 2015 PMID: 26315676, Binlie Yang et al. 2015 PMID: 26111245 |
| Modulate Mitochondrial Metabolism to Impair Cancer Cell Survival | Bela Ozsvari et al. 2017 PMID: 29080556, M Cazzaniga et al.2015 PMID: 26605341, Sonia M. et al. 2020 PMID: 32818805, Sas N. D. et al. 2020 PMID: 32152409 |
| Activate Apoptotic Pathways in Malignant Cells | H. Song et al. 2014 PMID: 25502932, D. Nicole et al. 2008 PMID: 18667591, J. Sasaki et al. 2002 PMID: 12479701, Neda Bayat et al. 2016 PMID: 27836464, Paola C. et al. 2005 PMID: 15705602, O. Fromigué et al. 2006 PMID: 16470222, K. Kalinsky et al. 2017 PMID: 27305912, Wen Jiang et al. 2021. PMID: 34303383 |
| Disrupt DNA damage repair pathways in cancer cells. | Elena V. E. et al. 2018 PMID: 29030460, Rebecca L. et al. 2015 PMID: 26087309, Maria Peiris-Pagès et al. 2015 PMID: 26425660 |
| Intervening with cancer stem cells may make them more receptive to standard oncological treatments. | Nipun Saini et al. 2018. PMID: 29342230, Jason R. B. et al. 2020 PMID: 32369446, Heather A. H. et al. 2009. PMID: 19752085, Cristian S. et al. 2018 PMID: 30364293, Yang Binlie. et al. 2015 PMID: 26111245, Neda Bayat et al. 2016 PMID: 27836464, S. Kato et al. 2018 PMID: 29848667, Liudmila L. K. et al. 2011 PMID: 21551187, Wen Jiang et al. 2021. PMID: 34303383, Anna M. et al. 2019 PMID: 31054863, |
| Suppress the development of tumor-associated blood vessels (anti-angiogenic effect) | Ren-Yuan Bai et al. 2015 PMID: 25253417, Stefania O. et al. 2015 PMID: 25196138, Weikun Qian et al. 2018 PMID: 30053447 |
Cancer Pathways/ Mechanisms Targeted by Natural Supplements
| Cancer Pathway / Mechanism | Natural Supplement | Source | (PubMed / Research Reference) |
| Inhibits PI3K/Akt/mTOR, NFκB, apoptosis | Curcumin | Turmeric | Maura C. et al. 2020 PMID: 32178382, Valeria C. et al. 2024. PMID: 39063939, Jessica M. et al. 2021 PMID: 34836091, |
| Modulates metabolism, induces apoptosis | Resveratrol | Grapes, Berries | Maura C. et al. 2020 PMID: 32178382, Valeria C. et al. 2024. PMID: 39063939, |
| Inhibits PI3K/Akt/mTOR, induces apoptosis | EGCG | Green Tea | V. Conti et al. 2024. PMID: 39063939, Jessica M. et al. 2021 PMID: 34836091 |
| Inhibits PI3K/Akt/mTOR, NFκB, apoptosis | Quercetin | Fruits, Veggies | V. Conti et al. 2024. PMID: 39063939, |
| DNA methylation, apoptosis, antioxidant | Selenium | Brazil nuts, fish | Michelle Harvie 2017 PMID: 24857143, María E. M. et al. 2012 PMID: 22534785, Oladapo F. F. et al. 2023 PMID: 37047063 |
| Modulates cell growth, nutrient signaling | Vitamin D | Sunlight, Diet | Michelle Harvie 2017 PMID: 24857143, W. H. Talib et al. 2024 PMID: 38274206, |
| Downregulates PI3K/Akt, apoptosis | Vitamin K2 | Leafy Greens | Oladapo F. F. et al. 2023 PMID: 37047063 |
| Anti-proliferative, pro-apoptotic | Polyphenols | Various Plants | Jessica M. et al. 2021 PMID: 34836091 |
